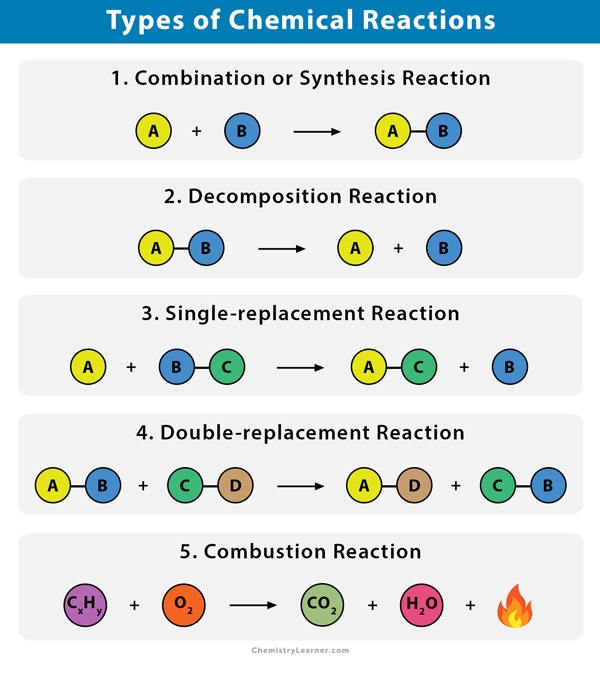
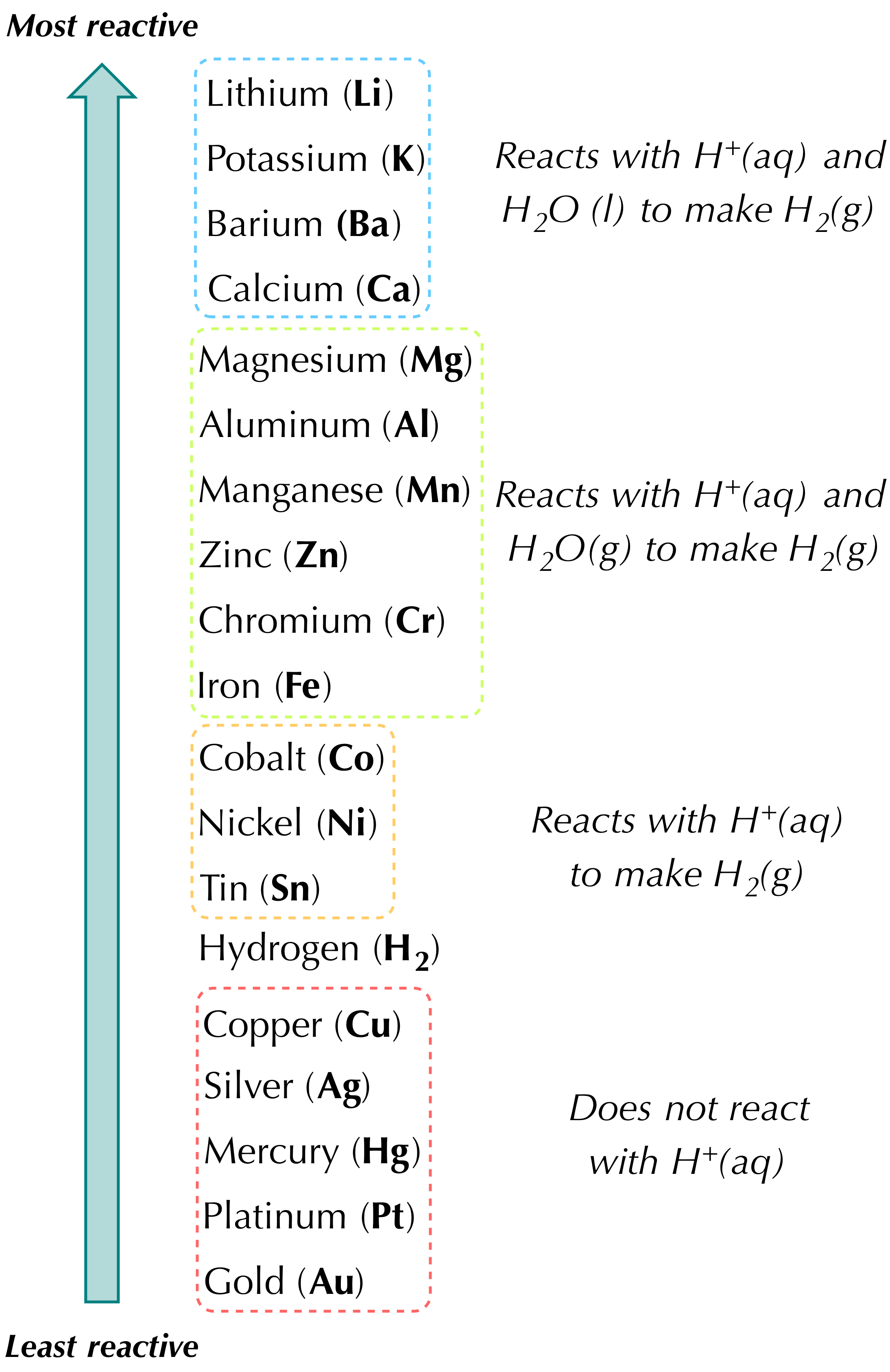
In Most Single Replacement Reactions a Metal Atom Replaces a
It generally takes the form of AX Y -- YX A or A XY -- XA Y. In a single replacement reaction either the anion negatively charged ion or the cation positively charged.
Module 4 Review Chemistry Quizizz
Draw two other possible isomers in which the chlorine atom replaces a different hydrogen atom attached to the aromatic ring.

. Solution Since the six-carbon ring with alternating double bonds is necessary for the molecule to be classified as aromatic appropriate isomers can be produced only by changing the positions of the chloro-substituent relative to the methyl-substituent. A single replacement reaction occurs when one element replaces another element within a compound. One reactant is always a single element and the other reactant is always a compound.

Single Replacement Reaction Definition And Examples
Single Replacement Reactions Article Khan Academy

How To Predict Products For Single Replacement Reactions Youtube
No comments for "In Most Single Replacement Reactions a Metal Atom Replaces a"
Post a Comment